Ecraid network responds to hypervirulent Klebsiella pneumoniae warning
The ECDC cautions that hypervirulent Klebsiella pneumoniae may be causing untreatable infections in Europe. The teams behind Ecraid's platform studies on ventilator-associated pneumonia and complicated urinary tract infections swiftly analysed their data to quantify the prevalence of carbapenem-resistant Klebsiella.
On 14 February, the European Centre for Disease Prevention and Control (ECDC) issued a risk assessment on the emergence of hypervirulent Klebsiella pneumoniae ST23 carrying carbapenemase genes in Europe, concluding that “there is a possibility of potentially untreatable infections in previously healthy adults”. It was further concluded that “prospective data collection on hvKp isolates, including epidemiological and clinical data on cases of infection, carriage and associated risk factors, would improve the understanding of national spread and transmission routes and determine the need for further surveillance.” Ecraid CEO Marc Bonten discussed the warning in a blog post.
Ecraid's response
In the Ecraid network there are two perpetual observational studies (POS) with prospective data collection of patients at risk of ventilator-associated pneumonia (VAP) (POS-VAP, led by the research team in Limoges in collaboration with Utrecht and Geneva) and with complicated urinary tract infection (cUTI) (POS-cUTI, led by the research team in Seville together with Utrecht and Verona). The teams immediately assessed the databases to quantify the potential prevalence of these carbapenem-resistant hypervirulent Klebsiella pneumoniae isolates in both studies.
As of 28 February, POS-VAP has enrolled 2,342 patients in 22 ICUs in 9 countries, of which 410 have developed VAP. POS-cUTI has enrolled 1,846 patients in 32 hospitals in 13 countries.
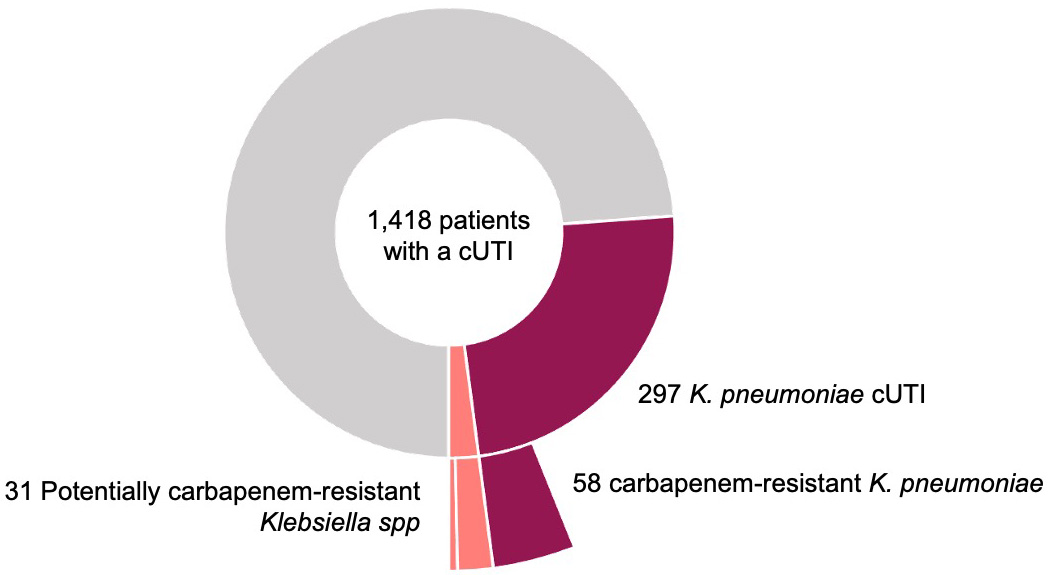
Figure 1. Prevalence of carbapenem-resistant Klebsiella in patients with a cUTI
POS-cUTI
Data in POS-cUTI were analysed from the first 1,418 patients, of which 297 (21%) had UTI-associated Klebsiella pneumoniae isolates, of which 58 (4% of all patients and 20% of Klebsiella pneumoniae associated UTI) were resistant to carbapenem. In addition, there were 25 patients with carbapenem resistant Klebsiella spp and 6 with Klebsiella spp not reported as carbapenem-resistant but treated with second line “carbapenem-free” treatments (aminoglycosides, tygecicline, colistin, ceftazidime/avibactam). Therefore, the potential prevalence of carbapenem-resistant Klebsiella among UTI patients was 6% (89 of 1418) (Figure 1).
The 58 carbapenem-resistant Klebsiella pneumoniae were identified in 8 of the 11 countries contributing to this dataset and 66% of the isolates came from 3 hospitals in Serbia where 68% of the Klebsiella pneumoniae isolates were resistant to carbapenem.
POS-cUTI lead Jesús Rodríguez-Baño praised the participating sites: "We were happy to see the positive response from sites participating in POS cUTI and to be able to provide data in case of alerts. Also, the team is willing to learn and adapt internally as a response to any medical need."
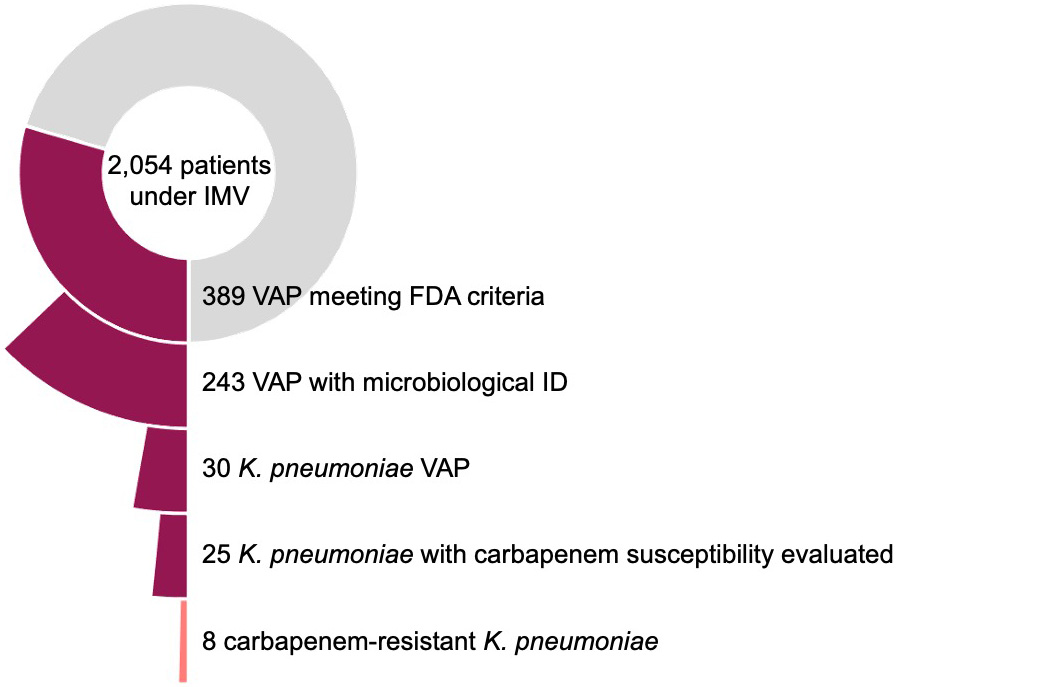
Figure 2. Prevalence of carbapenem-resistant Klebsiella in patients with microbiologically confirmed VAP
POS-VAP
The analysis of the POS-VAP study included 2,054 patients under invasive mechanical ventilation. Overall, 389 (19%) patients met FDA diagnostic criteria of VAP, of which 243 (10%) had microbiologic confirmation: 30 patients had Klebsiella pneumoniae VAP; that is 12% of those with microbiological confirmation, 8% of all episodes of VAP, and 1.5% of all patients at risk for VAP. Carbapenem susceptibility was assessed in 25/30 patients and detected in 8 (32%) from patients in ICUs in 3 out of the 9 reporting countries, which included countries not yet on the ECDC list. So, the prevalence of carbapenem-resistant Klebsiella for patients with microbiologically confirmed VAP was 3% (8 of 243) (Figure 2).
More details from patients and isolates will be collected and analysed. The preliminary conclusion is that the prevalence of carbapenem-resistance in Klebsiella pneumoniae is 6% among patients with complicated UTI and 3% among patients with microbiologically confirmed VAP. Whether these isolates belong to the hypervirulent Klebsiella pneumoniae ST23 type remains to be determined.
POS-VAP medical coordinator Ana Hernandez reflects on the legacy of the COVID-19 pandemic in the context of current threats, such as Klebsiella pneumoniae: "In 2020, the real-life availability of data on testing, diagnosis and typification of new SARS-COV2 cases democratised epidemiological data, helping to raise awareness about the importance of preventive measures among the general public and policymakers. Access to continuously updated information undoubtedly played a role on the successful public health management of the COVID pandemic, and this strategy should be used to tackle ‘the silent pandemic’ of antimicrobial resistance."
POS-VAP and POS-cUTI are being conducted under the ECRAID-Base project which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 965313.