Why data management matters
Data management in the context of clinical research refers to the organised and systematic handling of data collected during clinical trials and studies. It encompasses various activities that ensure the accuracy, integrity, security, and accessibility of clinical research data, as well as compliance with regulations like ICH-GCP and GDPR.
Data management is a critical aspect of clinical research, as the quality of the data directly impacts the validity and reliability of study results. Within the ECRAID-Prime project, this aspect is undertaken by Frank Leus and his team from the University of Utrecht, who ensure that appropriate data security and privacy measures are in place to facilitate complete, consistent and high-quality data collection for the study.
Ensuring data security and quality
Data management begins with the collection of information from study participants, and this can include medical records, laboratory test results and patient-reported outcomes, among others.
Lisanne Vintcent, who is the data manager of the ECRAID-Prime study, emphasises the importance of safeguarding patient data. She explains, “If our participant data is not sufficiently secured, in the event of a data breach, there is a risk that sensitive data will end up on the streets. This is of course unacceptable for the participants involved. Furthermore, under the European Union’s General Data Protection Regulation (GDPR), study participants have the right to control their own data. Failure to properly secure and process participant-related data can severely impact the study’s integrity and outcome.”
As Frank, who has extensive experience in coordinating data management of large-scale multi-centre observational and interventional clinical studies, succinctly puts it, “Rubbish in, rubbish out. If the quality of the data collected is poor, you cannot guarantee reliable research conclusions.”
To ensure data quality, Frank and his team employ an easy-to-use electronic data collection system that complies with regulations, incorporates extensive data entry checks in the electronic case report form (eCRF) and works in tandem with study monitors as well as research nurses to validate, clean and complete the collected data.
Data management goals
Within the EU-funded project, the work package’s primary objectives are to develop the Data Management Plan (DMP) which has been completed and serves as the foundation for all data management-related activities. This includes the development and implementation of data management and collection tools, processes and standard operating procedures (SOPs) for the study. The team is involved throughout the study to ensure a complete, correct and consistent database for analyses.
“Rubbish in, rubbish out. If the quality of the data collected is poor, you cannot guarantee reliable research conclusions.” - Frank Leus.
Since December 2021, the team has made substantial progress, including the implementation and testing of various data management systems for data collection. Next to this, they work closely with consortium partner, the European Clinical Research Infrastructure Network (ECRIN) to establish procedures, instruments and conditions for sharing the collected data with investigators of other projects.
Next to this, they work closely with consortium partner, the European Clinical Research Infrastructure Network (ECRIN) to establish procedures, instruments and conditions for sharing the collected data with investigators of other projects.

Cross-collaboration across work packages
The team maintains close coordination with the other work packages (WPs) in the project. Lisanne shares, “We work closely with WP2 (Trial) to define and test data collection systems, and WP3 (Lab) for sample data flow. We are also collaborating with the University of Oxford’s statistical team, as well as our system suppliers – Sortition for patient randomisation, Castor for eCRF collection, and YourResearch for sharing data between general practitioners and their national coordinating teams to enable the follow-up of participants.”
In the meantime, the team is working on standardising the eCRF by mapping all variable names to CDASH, a standard designed to improve data interoperability, in compliance with FAIR (Findable, Accessible, Interoperable, and Reusable Data) data principles. This is important so that the data collected within the ECRAID-Prime, as well as ECRAID-Base projects, are CDASH standardised and as prospectively harmonised as possible, so that it is easier to, for example, pool and compare data from different studies.
Improving user experience
Next to this, the team is actively developing a suite of tools aimed at improving the overall user experience. This includes an information manual for study monitors and research nurses, complete with clear instructions for navigating and utilising the project’s different data collection systems, as well as a patient-centric online diaries system to facilitate ease of use.
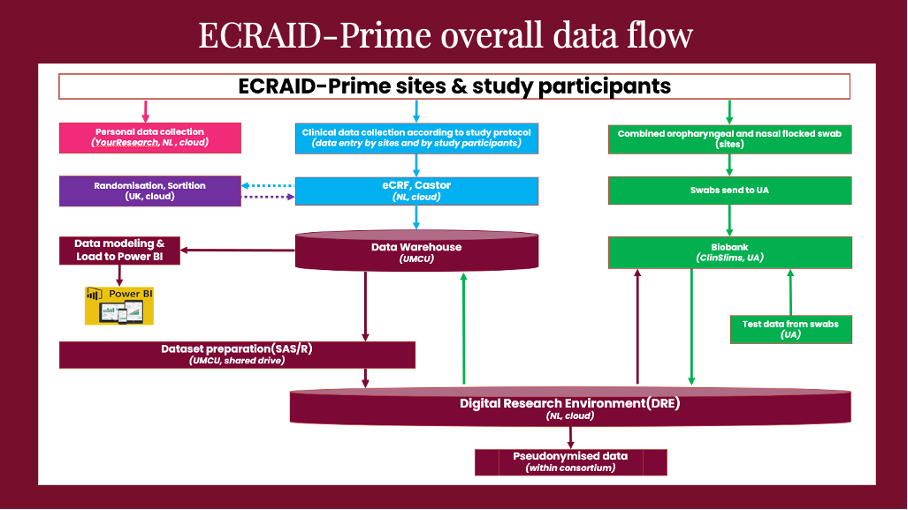
Lisanne adds, “When the study is up and running, we will introduce real-time dashboards using Power BI visuals. These dashboards will serve as invaluable tools for monitoring overall recruitment rates, our general practitioners’ performance, and ensuring the quality of collected data.”
Continuous data improvements
Looking ahead, the team is committed to further enhance data management processes within the dynamic ECRAID-Prime adaptive platform trial.
Frank states, “From the data management perspective, the ability to rapidly and seamless add and integrate new study interventions into our adaptive platform trial is vital for our success. So is the continuous enhancement of our patient inclusion and data collection processes to create a seamless, user-friendly experience for our general practitioners and participants, for example, through innovative solutions such as e-consent forms and mobile apps.”
“In essence, we strive to build a future where data management is a smooth and seamless process, and the overall experience for everyone involved is easy and effortless, as we introduce new treatments for COVID-19 and COVID-like illness.”
+ Read more about the ECRAID-Prime adaptive platform trial design.